Background: Varnimcabtagene autoleucel (IMN-003A) is an anti-CD19 CAR-T cell immunotherapy for patients with relapsed refractory B cell malignancies (RR BCM) in the Phase 2 IMAGINE study. Hypogammaglobinemia can result from normal CD19+ B cell depletion (on-target, off-site effect) by CD19 directed CAR-T cells. RR BCM patients (pts) are already at risk for infections due to impaired immune function, lymphodepletion preparative regimen and prior immunosuppressive therapies. Intravenous immunoglobulin (IVIg) replacement is used to reduce the risk of infections due to hypogammaglobulinemia. We report the impact of var-cel on hypogammaglobinemia, infectious complications, risk factors, IVIg usage and clinical outcomes for RR BCM patients treated in first-in-India industry study (CTRI/2022/03/041162).
Methods: RR BCM pts treated with var-cel in this study were reviewed. Hypogammaglobulinemia was defined as IgG <4 g/L. Infections reported during pre-apheresis (post screening), D0 to D+28 post CAR-T infusion and beyond D+29 for study duration were analyzed. IVIg usage for hypogammaglobulinemia was at investigator discretion. Patients were monitored for infection events. RR BCM response was assessed as per NCCN (B-ALL) and IWG (B-NHL) criteria. Appropriate statistical tools were used for data analysis.
Results: At data cut-off, of 25 pts enrolled (median age 31 yrs, range 3 - 66) with RR BCM (n=13 B-ALL; n=12 B-NHL), 24 pts received var-cel (1 withdrawal). Median follow-up after IMN-003A administration was 205 days (range 12 - 434). Overall response rate (ORR) was 91.7% (22/24) at D+28 (B-ALL 91.7%; B-NHL 91.7%) and 80.9% (17/21) at D+90 (B-ALL 80%; B-NHL 81.8%). Treatment related mortality was 4.2% (n=1/24); 3 pts died of disease progression.
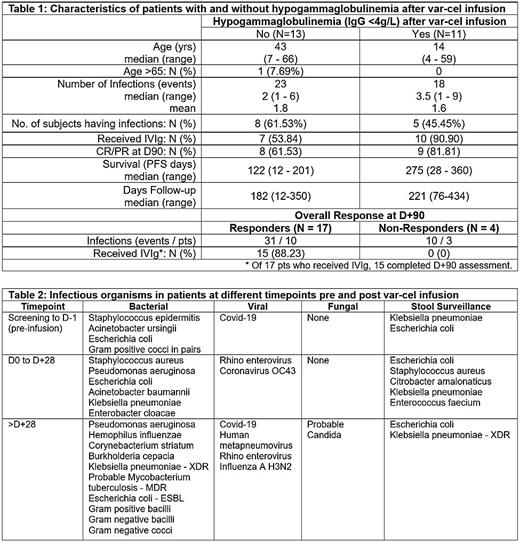
Of the 24 pts, none had hypogammaglobulinemia (IgG <4g/L) at baseline. Eleven pts (45.8%) developed hypogammaglobulinemia post var-cel infusion. Of these, 10 pts (90.9%) received prophylactic intravenous immunoglobulin (IVIg). In total, 70.8% (n=17/24) pts received IVIg infusions (Table 1).
The median time to initiation of IVIg after var-cel infusion was 58 days (range 15 to 189 days). Of evaluable pts, IVIg was used in 15 of 17 (88.2%) responders at D+90 (CR / PR) vs 0 of 4 (0%) in non-responders (SD / PD) (p=0.06). 52.9% (n=9/17) pts on prophylactic IVIg reported infections. Patients who received IVIg had more infections (30 events; p=0.76). The mean number of infections with and without IVIg was 1.8 and 1.6 respectively. The median overall survival was significantly higher in pts who received IVIg prophylaxis (p=0.02). There was no difference in survival observed based on presence or absence of hypogammaglobulinemia post var-cel infusion (p=0.46).
A total of 41 infections were reported in 13 pts. Of these, the incidence from screening to D-1; D0 to D+28 and beyond D+29 were 9 events (4 pts); 12 (11) and 20 (7) respectively. Bacterial, viral and fungal infections reported were 28 events (11 pts); 12 (8) and 1 (1) respectively. Bacterial infections were sepsis (n=7); urinary tract infections (n=5), respiratory (n=14) and central line (n=2). There were 12 viral events, 11 of respiratory origin and one involving conjunctiva. There was one episode of probable fungal candidemia. Stool surveillance detected 27 positive events in 13 pts. Table 2 summarizes the reported infectious organisms.
There were 23 infections documented in 13 patients who did not develop hypogammaglobulinemia (median 2 infections; mean 1.8; range 1 - 6). Of 11 pts with hypogammaglobulinemia, there were 18 infections (median 3.5; mean 1.6; range 1 - 9). Complete and partial responders had more infections compared to non-responders (p = 0.05).
Conclusions: This is the first-in-India report evaluating hypogammaglobulinemia and infection risk post CAR-T cell infusion. The IVIg usage and documented infections was more in responders. The safety and efficacy outcomes of varnimcabtagene autoleucel (IMN-003A) are consistent with reported outcomes of approved CD19 directed CAR-T cell therapies. The use of prophylactic IVIg, often led by individual practice, to reduce the number of infections and its impact on efficacy outcomes remains uncertain. Potential strategies to reduce infection risk may help improve outcomes.
Disclosures
B:Immuneel Therapeutics Private Limited: Current Employment. Arasu:Immuneel Therapeutics Private Limited: Current Employment. Elluru:Immuneel Therapeutics Private Limited: Current Employment. Akheel:Immuneel Therapeutics Private Limited: Current Employment. Kamat:Immuneel Therapeutics Private Limited: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal